Hypotonic Solution: Imagine you and two other people are waiting for an elevator in the lobby of a building. When the elevator doors open, you see there are 20 people crammed into that tiny space. Who in their right mind would try and fit on that elevator? Not you! There are just too many people there. The doors close, and you wait for the next one. This is basically what happens when two solutions meet in biology – the two solutions compare their respective crowdedness to each other, and then each solution reacts accordingly.
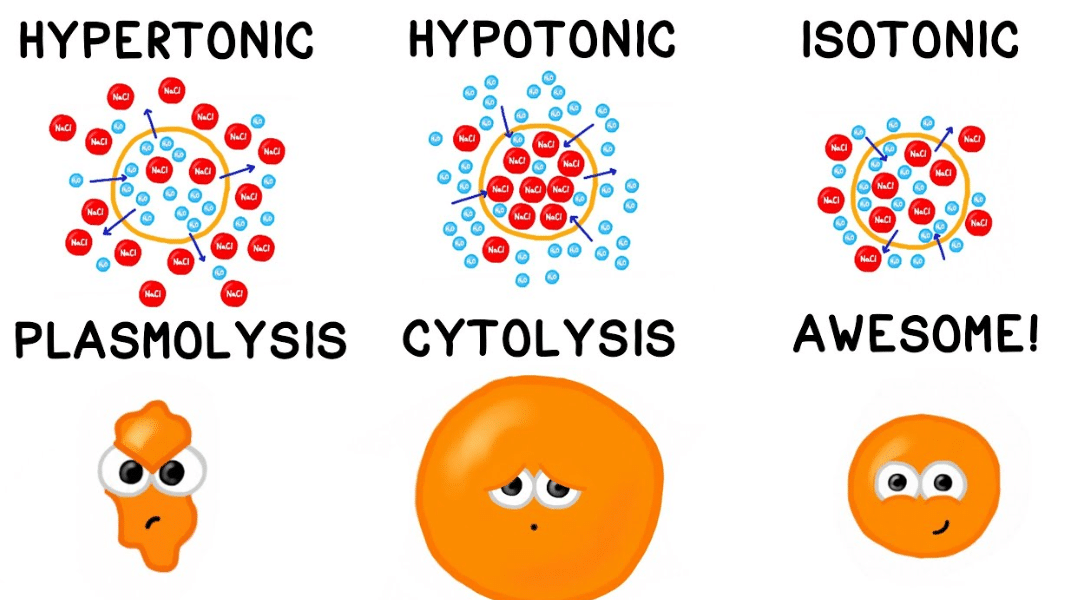
Sometimes, one solution has more ‘stuff’ crammed into it than the other. We call this stuff solute. The amount of solute in a solution determines how that solution will react when in the presence of another solution. A solution can be labeled one of three ways when it is compared to another solution. First, it may be considered an isotonic solution, meaning it has an equal amount of solute and water when compared to another solution. Second, it may be considered a hypertonic solution, meaning it has more solute and less water than another solution.
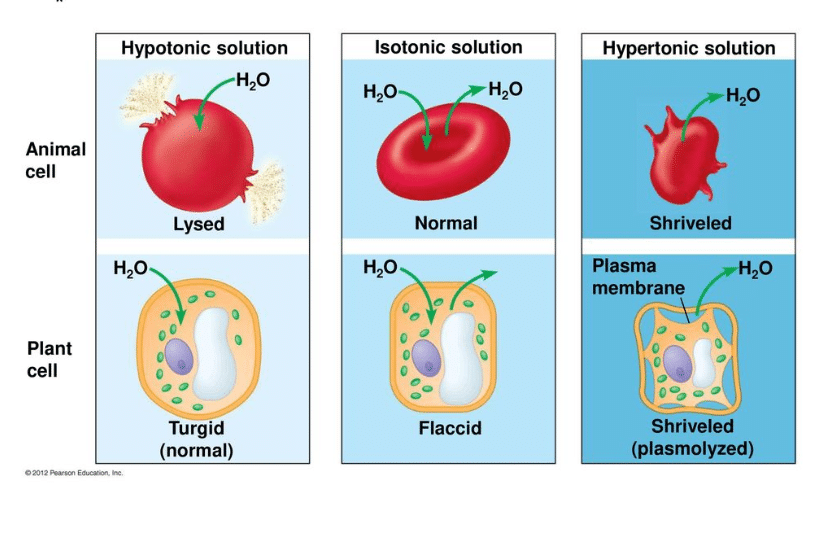
Third, it may be considered a hypotonic solution, meaning it has less solute and more water than another solution. This is the situation described above – when you were waiting in the lobby, you and the two other people were like solute in a solution, and all that space around was the water. When the elevator doors opened, there were a lot more people in a lot less space in that elevator – a lot more solute in a lot less water. Therefore, your lobby was considered hypotonic when compared to the cramped elevator.
How It works
This is actually a complicated question. To answer it, let’s take a step back and refresh our memory on why diffusion happens. In diffusion, molecules move from a region of higher concentration to one of lower concentration—not because they’re aware of their surroundings, but simply as a result of probabilities. When a substance is in gas or liquid form, its molecules will be in constant, random motion, bouncing or sliding around one another.
If there are lots of molecules of a substance in compartment A and no molecules of that substance in compartment B, it’s very unlikely—impossible, actually—that a molecule will randomly move from B to A. On the other hand, it’s extremely likely that a molecule will move from A to B. You can picture all of those molecules bouncing around in compartment A and some of them making the leap over to compartment B. So, the net movement of molecules will be from A to B, and this will be the case until the concentrations become equal.
Read Also: What Is Indirect Characterization? Examples
In the case of osmosis, you can once again think of molecules—this time, water molecules—in two compartments separated by a membrane. If neither compartment contains any solute, the water molecules will be equally likely to move in either direction between the compartments. But if we add solute to one compartment, it will affect the likelihood of water molecules moving out of that compartment and into the other—specifically, it will reduce this likelihood.
Hypotonic Solution Examples
Plants and Fungi
Large plants and fungi control the environment around their cells, helping ensure the environment is always a hypotonic solution, compared to the cells. This creates cells that are turgid. The turgid cells push outward on their cell walls, which push against each other creating a rigid structure. The organisms are constantly cycling solutes, to keep the contents of their cells filled with water. If you’ve ever over-fertilized your garden, you know it is not good for plants. The added solutes in the soil turn the hypotonic solution around the roots into a hypertonic solution. Thus, the roots and the entire plant are quickly drained of water. Organisms in this condition will quickly die because they cannot complete the reactions necessary to sustain life.
Animal Cells
Animal cells do not have a cell wall. Typically, animals rely on their skin to separate the outside environment from their inside organs. The fluid inside of their body cavity can then be regulated by a series of membranes and proteins. The fluid will thus remain an isotonic or slightly hypotonic solution in comparison to the cells, keeping them plump and healthy without destroying them. The process of maintaining the solute concentration in an organism is known as osmoregulation and occurs in all animals. Many animals that live in the ocean have salt glands which expel excess salt from their body. The animals must drink the salt water to get the water into their bodies, but the salts must be concentrated and excreted from the body to maintain it as a hypotonic solution.
Read Also: What Is Computer Architecture?
Hypotonic IV Solution
The Dextrose solutions come in a variety of concentrations; 2.5% (hypotonic), 5% (isotonic), 20% and 50% (hypertonic). These solutions provide both fluids and carbohydrates for energy and thus prevent the breakdown of fats and proteins for energy. The amount of fluid/carbohydrate is dependent on the solution.
The osmolarity of a dextrose solution is different than with other types of IV solutions because once the dextrose is metabolized the osmotic effect changes. In other words, these dextrose solutions have a different physiological osmolarity than they do in the IV bottle. As the dextrose is metabolized, free water remains for hydration.
Read Also: What Are The Agents Of Socialization? Example