Concentration Gradient | What Is A Concentration Gradient
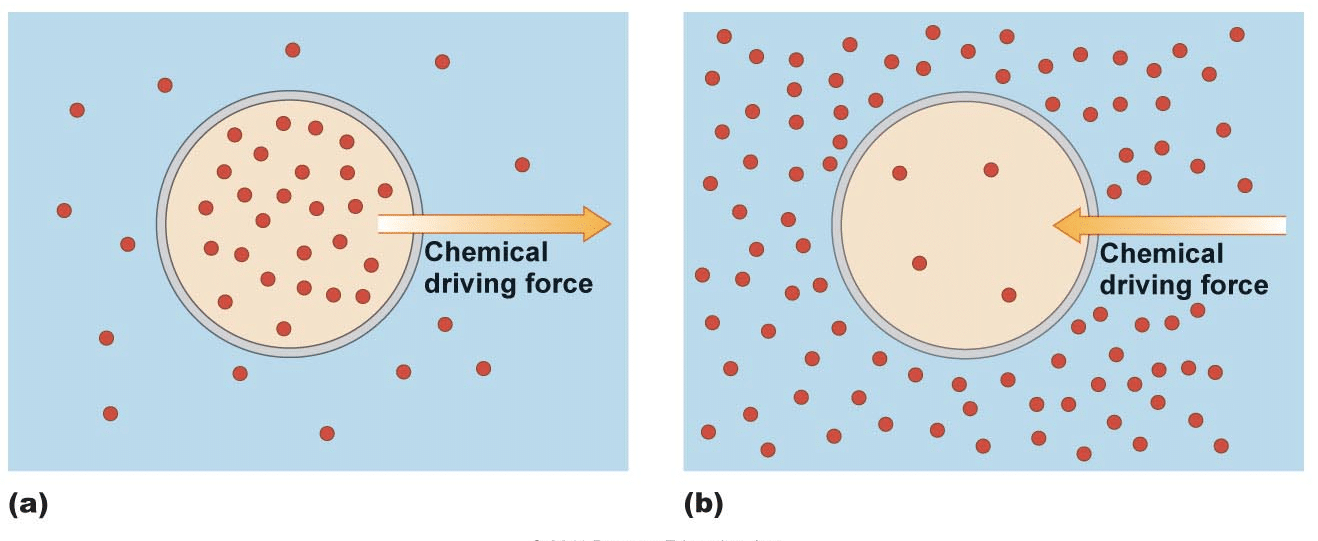
The formal definition of a concentration gradient is the process of particles, which are sometimes called solutes, moving through a solution or gas from an area with a higher number of particles to an area with a lower number of particles. The areas are typically separated by a membrane. This membrane can be permeable, semi-permeable, or non-permeable. Permeable is defined as a membrane that can be crossed by particles, ions, or water. Semi-permeable means that some particles, ions, or water can cross the membrane. Finally, a non-permeable membrane means that no particles, ions, or water can cross the membrane.
An example that might help you understand the different types of membranes would be different types of fences. A wooden log fence would allow many things to pass through – this would be an example of a permeable membrane. A chain-link fence would allow some small items to pass through it – this would be like a semi-permeable membrane. A solid plastic fence would not allow items to pass through it at all – this would represent a non-permeable membrane.

The function of Concentration Gradients
Concentration gradients are a natural consequence of the laws of physics. However, living things have found many ways to use their properties to accomplish important life functions. Organisms that need to move a substance in or out of their cells, for example, may use the movement of one substance down its concentration gradient to transport another substance in tandem.
Organisms may also use concentration gradients to accomplish sudden changes or movements by releasing high concentrations of solute to move to low-concentration areas. Neurons are an example of cells that use high concentrations of solutes to accomplish rapid changes.
Everyday Examples of Concentration Gradient
The concentration of scent molecules is highest on areas of the skin that have had perfume or aftershave directly applied. Others can smell the scent because some of those molecules are always traveling away from the perfumed person, the source, out into the air—moving down the concentration gradient, from a high concentration to a lower concentration. Eventually, the scent molecules are so widely dispersed that they can no longer be detected.
Think about the unpleasant scent of skunk stink, when a skunk has sprayed or been struck by a vehicle. As a person gets closer to the source of the skunk stink, the scent gets stronger, because the “stink molecules” are more highly concentrated closer to the source.
Molecules not only travel through the air but through other media as well. When a person puts creamer in his or her coffee, the cream molecules would eventually bounce around in the cup, moving down the concentration gradient until evenly distributed. However, most coffee drinkers do not wait for this to happen. They introduce additional energy by stirring the coffee and speeding up the process.
Concentration Gradient Definition Biology
In cell biology, diffusion is a main form of transport for necessary materials such as amino acids within cells.[1] The diffusion of solvents, such as water, through a semipermeable membrane, is classified as osmosis.
Metabolism and respiration rely in part upon diffusion in addition to bulk or active processes. For example, in the alveoli of mammalian lungs, due to differences in partial pressures across the alveolar-capillary membrane, oxygen diffuses into the blood and carbon dioxide diffuses out. Lungs contain a large surface area to facilitate this gas exchange process.
What Does A Concentration Gradient Have To Do With A Random Walk?
Remember the biased random walk? Well, there’s always a reason for bias. Bacteria can bias their walks based on the concentration gradient of a particular chemical. So even though each step is in a random direction, the length of the step is longer if the bacterium is moving towards a higher concentration than it is if the bacterium is moving towards a lower concentration.
Let’s watch the biased random walk video again, this time with the concentration gradient in the background. Now you can see the reason for the bias in the walk!
How Can Bacteria Tell If They’Re Moving Towards Higher Or Lower Concentration?
When a bacterium is looking for a particular chemical signal, it detects this chemical as it moves along its path. If it is moving up the concentration gradient, it will start detecting the chemical molecules more and more frequently. If it is moving down the concentration gradient, it will start detecting the chemical molecules less and less frequently. This ultimately determines the direction and strength of the bias in its random walk.
Non-equilibrium System
Because chemical diffusion is a net transport process, the system in which it takes place is not an equilibrium system (i.e. it is not at rest yet). Many results in classical thermodynamics are not easily applied to non-equilibrium systems. However, there sometimes occur so-called quasi-steady states, where the diffusion process does not change in time, where classical results may locally apply. As the name suggests, this process is not a true equilibrium since the system is still evolving.
Non-equilibrium fluid systems can be successfully modeled with Landau-Lifshitz fluctuating hydrodynamics. In this theoretical framework, diffusion is due to fluctuations whose dimensions range from the molecular scale to the macroscopic scale.
Chemical diffusion increases the entropy of a system, i.e. diffusion is a spontaneous and irreversible process. Particles can spread out by diffusion, but will not spontaneously re-order themselves (absent changes to the system, assuming no creation of new chemical bonds, and absent external forces acting on the particle).
Read Also: Antiderivative Calculator – A Brief Introduction