Empirical Formula: Once the experimental formula is found, the molecular formula for a compound can be determined if the molar mass of the compound is known. Simply forecast the mass of the empirical creed and divide the molar mass of the compound by the mass of the empirical formula to find the ratio between the molecular formula and the empirical creed. Multiply all the atoms (among) by this ratio to find the molecular creed. In chemistry, the empirical formula of a chemical compound is the simplest productive integer ratio of atoms present in a compound.
A simple example of this concept is that the empirical creed of sulfur monoxide, or SO, would simply be SO, as is the experimental formula of disulfur dioxide, S2O2. Thus, sulfur monoxide and disulfur dioxide, both compounds of sulphur and oxygen, have the same empirical formula. However, their atomic formulas, which express the number of atoms in each molecule of a chemical compound, are not the same.
Empirical Formula Calculator
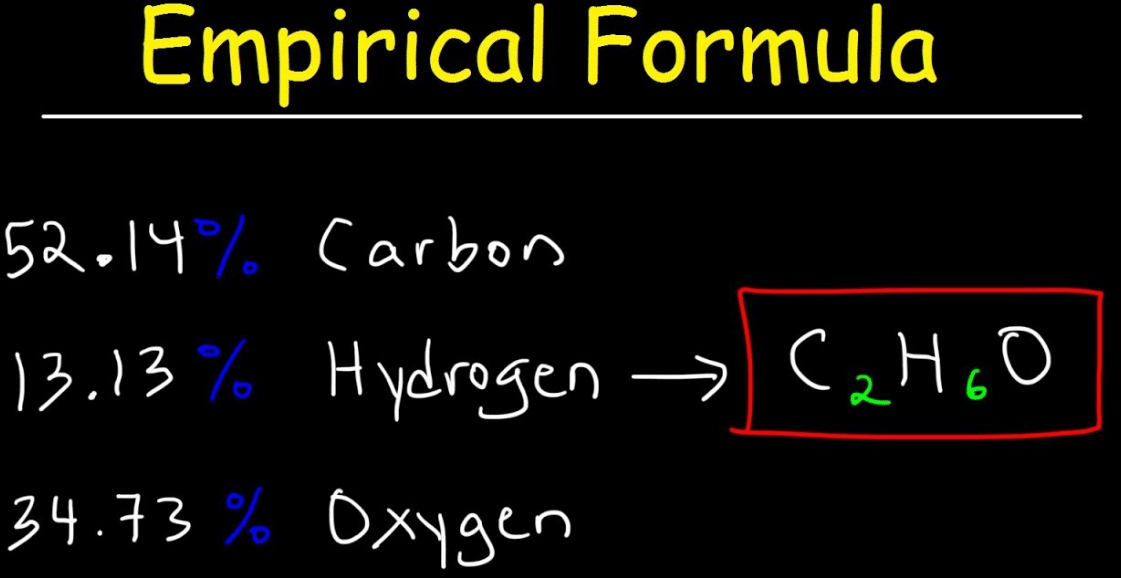
The empirical creed of a compound is the straightforward whole number ratio of each type of atom in a compound. It can be the same as the compound’s molecular creed, but not always. An empirical creed can be calculated from instruction about the mass of each element in a commixture or from the percentage composition.To calculate the experimental formula, you must first determine the relative masses of the different elements present. You can either use mass data in grams or percent composition.
For percent balance, we assume the total percent of a commixture is equal to 100% and the percent formation is the same in grams. For example, the total mass of the commixture is 100 grams. If a commixture contained 68% carbon, 9% hydrogen, and 23% oxygen, we would assume 68 grams of carbon, 9 grams of hydrogen, and 23 grams of oxygen.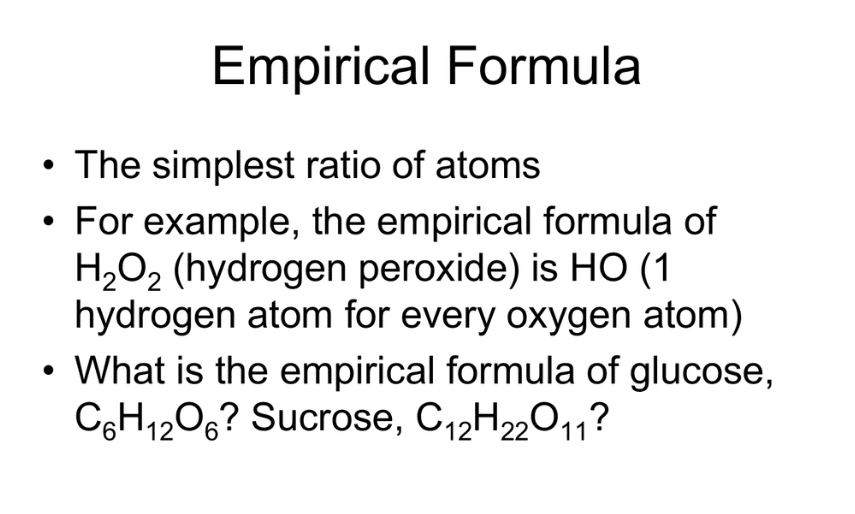
Empirical Formula CalculatorThe steps for determining the empirical formula of a compound are as follows:
Step 1: Obtain the mass of each element present in grams
Element % = mass in g = m
Step 2: Determine the number of moles of each type of atom present
m/atomic mass = Molar amount (M)
Step 3: Divide the number of moles of each element by the smallest number of moles
M / least M value = Atomic Ratio (R)
Step 4: Convert numbers to whole numbers. This set of whole numbers is the subscripts in the empirical formula.
R * whole number = Empirical Formula
How To Find Empirical Formula
You can use the experimental formula to find the atomic formula if you know the molar mass of the compound. To do this, calculate the empirical formula mass and then divide the compound molar throng by the empirical formula mass. This gives you the ratio between the atomic and empirical formulas. Multiply all of the subscripts in the empirical formula by this ratio to get the indexes for the molecular creed.
Here’s how to find an empirical formula when given percent composition:
-
Assume that you have 100 g of the unknown compound.
The beauty of this little trick is that you conveniently gift yourself with the same number of grams of each elemental component as its contribution to the percent composition. For example, if you assume that you have 100 g of a compound composed of 60.3% magnesium and 39.7% oxygen, you know that you have 60.3 g of magnesium and 39.7 g of oxygen. (The only time you don’t do this is if the problem specifically gives you the masses of each element present in the unknown compound.)
-
Convert the masses from Step 1 into moles using the molar mass.
-
Determine which element has the smallest mole value. Then divide all the mole values you calculated in Step 2 by this smallest value.
This division yields the mole ratios of the elements of the compound.
-
If any of your mole ratios aren’t whole numbers, multiply all numbers by the smallest possible factor that produces whole-number mole ratios for all the elements.
For illustration, if you have 1 nitrogen atom for every 0.5 oxygen atoms in a compound, the experimental formula is not N1O0.5. Such a creed casually recommends that an oxygen atom has been split, an object that would create a small-scale nuclear explosion. Though impressive-sounding, this scheme is almost certainly false. Far more reasonable is that the atoms of nitrogen and oxygen are incorporating in a 1: 0.5 ratio but do so in a larger but comparable ratio of 2: 1. The empirical formula is thus N2O.
Because the original percent composition data is typically experimental, expect to see a bit of error in the numbers. For example, 2.03 is probably within experimental error of 2, 2.99 is probably 3, and so on.
-
Write the empirical formula by attaching these whole-number mole ratios as subscripts to the chemical symbol of each element.
Order the elements according to the general rules for naming ionic and molecular compounds.
Empirical Vs Molecular Formula
The empirical formula of a compound is defined as the formula that shows the ratio of elements present in the compound, but not the actual numbers of atoms found in the molecule. The ratios are denoted by subscripts next to the element symbols.
Also Known As: The empirical formula is also known as the simplest formula because the subscripts are the smallest whole numbers that indicate the ratio of elements.
Empirical Formula Examples
Glucose has a molecular formula of C6H12O6. It contains 2 moles of hydrogen for every mole of carbon and oxygen. The empirical formula for glucose is CH2O.
The molecular formula of ribose is C5H10O5, which can be reduced to the empirical formula CH2O.
How to Determine Empirical Formula
- Begin with the number of grams of each element, which you usually find in an experiment or have given in a problem.
- To make the calculation easier, assume the total mass of a sample is 100 grams, so you can work with simple percentages. In other words, set the mass of each element equal to the percent. The total should be 100 percent.
- Use the molar mass you get by adding up the atomic weight of the elements from the periodic table to convert the mass of each element into moles.
- Divide each mole value by the small number of moles you obtained from your calculation.
- Round each number, you get to the nearest whole number. The whole numbers are the mole ratio of elements in the compound, which are the subscript numbers that follow the element symbol in the chemical formula.
Sometimes determining the whole number ratio is tricky and you’ll need to use trial and error to get the correct value. For values close to x.5, you’ll multiply each value by the same factor to obtain the smallest whole number multiple. For example, if you get 1.5 for a solution, multiply each number in the problem by 2 to make the 1.5 into 3. If you get a value of 1.25, multiply each value by 4 to turn the 1.25 into 5.
What is the empirical formula of c2h2?
How do you find the empirical formula of a compound?
What is the empirical formula of the compound? Start with the number of grams of each element, given in the problem. Convert the mass of each element to moles using the molar mass from the periodic table. Divide each mole value by the smallest number of moles calculated.
What is the empirical formula for the molecule c6o2h6?